-
The University
- Welcome
- Who we are
- Media & PR
- Studying
-
Research
- Profile
- Infrastructure
- Cooperations
- Services
-
Career
- Med Uni Graz as an Employer
- Educational Opportunities
- Work Environment
- Job openings
-
Diagnostics
- Patients
- Referring physicians
-
Health Topics
- Health Infrastructure
Research team Pritišanac
Research focus: Inflammation, autoimmunity and cancers
Approximately 60% of human proteins contain lengthy segments lacking stable secondary or tertiary structures, known as intrinsically disordered protein regions (IDRs). These regions have received increasing recognition as key regulators across a spectrum of cellular processes, including mRNA transcription, splicing, protein translation, signaling, and localization to specific biomolecular condensates. Moreover, IDRs are enriched in risk genes that are associated with complex diseases, including neurodevelopmental disorders and cancer (Tsang, Pritišanac et al. 2020 Cell). To execute their functions, IDRs engage in intra- and inter-molecular interactions that are typically transient and highly dynamic, and can adjust their interface depending on the binding partner (Alderson & Pritišanac, et al. 2023 PNAS).
Our research aims to deepen the understanding of molecular recognition in the dynamic regions of the proteome and transcriptome. By combining computational structural biology, cutting-edge AI models, bioinformatics, and protein NMR spectroscopy, we seek to understand, predict, and design tunable biomolecular interactions crucial for health and disease pathology. Together with our collaborators, we conduct research that spans basic science, drug discovery, interpretation of disease variants, and protein engineering.
Network: In addition to the collaboration within the Otto-Loewi Research Center and MUG, we join forces with researchers from around the world. Our collaborators include Reid Alderson (Helmholtz Munich), Lu-Yang Wang (SickKids Toronto), Jason Moffat (University of Toronto), Anne Conibear (TU Wien), Vaclav Veverka (Charles University), and Matteo Degiacomi (Durham University).
Division of Molecular Biology and Biochemistry
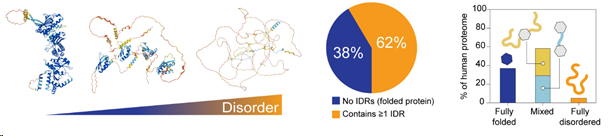
Individual proteins exist on a spectrum of intrinsic structural disorder (left). In the human proteome, over 60% of the proteins contain at least one intrinsically disordered region (middle). Disordered regions can occur either as termini that flank structured domains, or as linkers between structured domains (right). A small percentage (~5%) of the human proteome are fully disordered proteins.