-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
August 2023
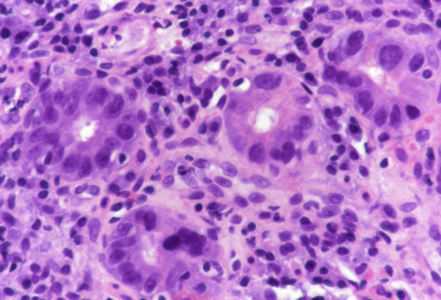
Duodenal biopsy in a 74-year-old male with dysphagia and history of sigmoid cancer.
Diagnosis
Chemotherapy-induced duodenitis.
Comment
A 74-year-old male with history of sigmoid cancer presented with dysphagia. Upon endoscopy, the mucosa of oesophagus and stomach appears entirely normal, while the duodenal mucosa shows patchy inflammatory changes. Biopsies are taken from the duodenum.
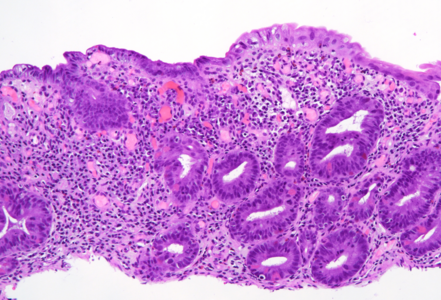
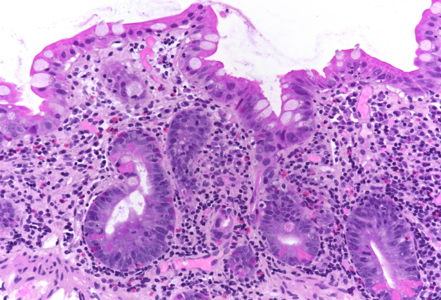
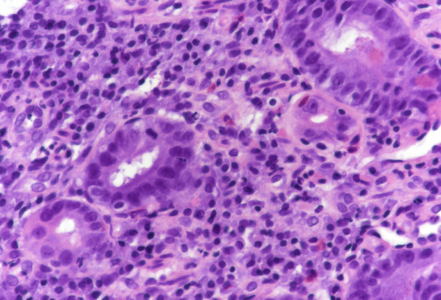
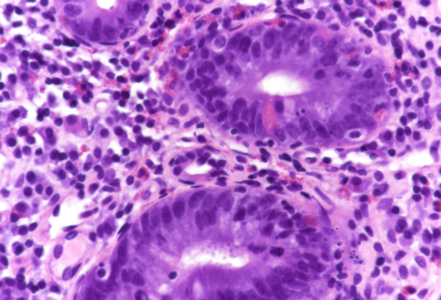
On low power, the architecture of the mucosa is altered, the crypts are irregular, displaying a mixture of crypt hyperplasia, but also crypt loss. Villous blunting is considerable, in parts the mucosa is entirely flat; no intraepithelial lymphocytosis (Panels A-B). The cell content within the stroma is increased, with many lymphocytes and plasma cells as well as striking overrepresentation of eosinophils, including degranulation and eosinophilic cryptitis (Panel C). The crypt epithelium is characterized by loss of goblet cells, increased apoptosis, nuclear plemorphism and focal cytoplasmic eosinophilia, thereby indicating cytopathic injury (Panels D-F). No pathological organisms are seen on the surface of the mucosa.
In all, the morphology argues in favour of a longer-lasting (“chronic”) mucosal injury, preferably by cytotoxic drugs. Considering the patient’s history, chemotherapy-induced duodenitis appears to be the diagnosis of choice, and is confirmed by clinical data: the patient is currently on folfox (folinic acid, fluorouracil, and oxaliplatin) plus avastin (bevacizumab) treatment for colonic cancer.
Chemotherapy-induced mucosal injury may affect all areas of the gastrointestinal tract, but is usually diagnosed within the large bowel, since endoscopy is often prompted by diarrhoea and/or haematochezia. Our patient did not report symptoms related to bowel injury, and material from the lower gastrointestinal tract was not available.
Upon histology, chemotherapy-induced duodenitis needs to be differentiated from other types of drug toxicity, inflammatory bowel disease affecting the upper gastrointestinal tract, but also celiac disease. Though crypt hyperplasia and nearly total villous atrophy are present, the features of cytopathic injury, that is, the various features of apoptotic enteropathy sive duodenopathy, in conjunction with stromal eosinophilia and lack of intraepithelial lymphocytosis, more or less exclude diagnosis of celiac disease and may help to avoid this important diagnostic trap.
For further reading
- Cerilli LA, Greenson JK. The differential diagnosis of colitis in endoscopic biopsy specimens: a review article. Arch Pathol Lab Med. 2012; 136: 854-64.
- Patil DT, Odze RD. Biopsy diagnosis of colitis: an algorithmic approach. Virchows Arch. 2018; 472: 67-80.
- Villanacci V, Vanoli A, Leoncini G, Arpa G, Salviato T, Bonetti LR, Baronchelli C, Saragoni L, Parente P. Celiac disease: histology-differential diagnosis-complications. A practical approach. Pathologica. 2020; 112: 186-196.
- Daum O, Daumová M, Švajdler M. Pattern-based approach to duodenitis and duodenopathy. Cesk Patol. 2022; 58: 88-99.
Presented by
Dr. Cord Langner, Graz, Austria.