-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
October 2023
Gastric biopsy in a 67-year-old male with peritoneal carcinomatosis.
Diagnosis
Prostate cancer metastasis to the stomach.
Comment
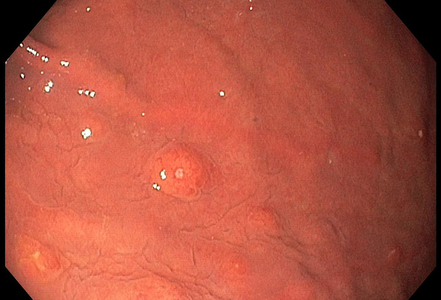
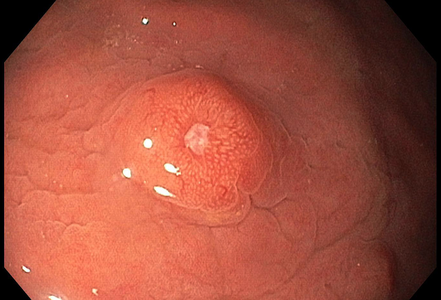
A 67-year-old male underwent endoscopic evaluation of the upper gastrointestinal tract after diagnosis of peritoneal carcinomatosis in the setting of unknown primary on CT scan (CUP syndrome). Within the gastric corpus, MULTIPLE nodular (sub)mucosal lesions, some of which with volcano-like central ulceration, were seen (Panels A-B). In addition to biopsy sampling, endoscopic mucosal resection (EMR) of the largest lesion was performed.
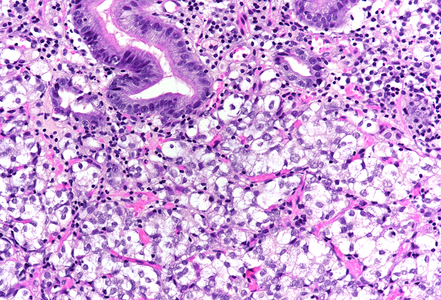
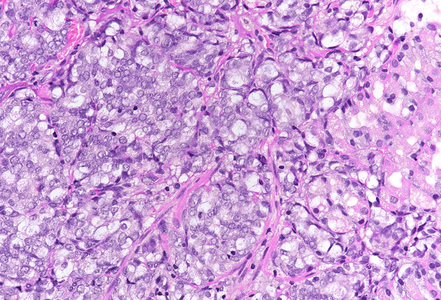
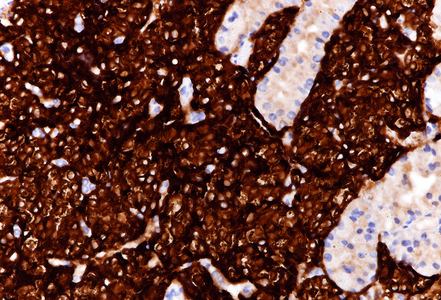
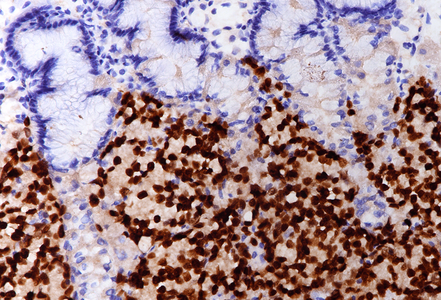
On low power, nodular infiltration of the corpus mucosa by middle-size pale to basophilic tumour cells is seen, arranged in solid nest-like arrangement lacking glandular appearance (Panel C-D). The surface epithelium is not involved, there is no background inflammation, atrophy and/or metaplasia. The tumour cells are positive for pan-keratin, keratin 8/18, however negative for keratins 7 and 20. This peculiar keratin profile prompted staining for Prostate-specific membrane antigen (PSMA, Panel E) and NKX3-1 which is an androgen-regulated, prostate-specific homeobox gene (Panel F). Ultimately, diagnosis of prostate cancer metastatic to the stomach was made.
Metastasis to the gastrointestinal tract is rare in general. Multiple lesions are more common than single lesions. Diagnosis may be challenging, in particular when the pathologist is unaware of the clinical setting, that is, the presence of widespread malignant disease.
Within the gastrointestinal tract, the stomach represents the organ most commonly affected by haematogenous cancer spread, whereas small bowel and large bowel are often found infiltrated directly from cancers in immediate vicinity, e.g., cancer invasion of the duodenal wall by pancreatic cancer or invasion of the rectum by prostate cancer. When metastasis to the stomach detected, it usually originates from malignant melanoma, (lobular) breast cancer, or kidney cancer (“the big three”), but virtually every primary cancer may be the source. Thus, several case reports of prostate cancer metastatic to the stomach are available in the literature (compare below).
For further reading
- Krones E, Stauber R, Vieth M, Langner C. Hypertrophic gastric folds caused by metastatic prostate adenocarcinoma. Endoscopy. 2012;44 Suppl 2 UCTN:E47-8.
- Mehrzad R, Agarwal A, Faller GT, Fiore JA. Prostate cancer metastasis to the stomach: 9 years after the initial diagnosis--case report and a literature review. J Gastrointest Cancer. 2014; 45 Suppl 1: 40-3.
- Inagaki C, Suzuki T, Kitagawa Y, Hara T, Yamaguchi T. A case report of prostate cancer metastasis to the stomach resembling undifferentiated-type early gastric cancer. BMC Gastroenterol. 2017; 17: 93. PMID: 28784100. Free PMC article.
- Gilg MM, Gröchenig HP, Schlemmer A, Eherer A, Högenauer C, Langner C. Secondary tumors of the GI tract: origin, histology, and endoscopic findings. Gastrointest Endosc. 2018; 88: 151-158.e1.
- Kaila V, Jain R, Lager DJ, Jensen P, Feldman M. Frequency of metastasis to the gastrointestinal tract determined by endoscopy in a community-based gastroenterology practice. Proc (Bayl Univ Med Cent). 2021; 34: 658-663. PMID: 34744302. Free PMC article.
- Moshref L, Abidullah M, Czaykowski P, Chowdhury A, Wightman R, Hebbard P. Prostate Cancer Metastasis to Stomach: A Case Report and Review of Literature. Curr Oncol. 2023 Mar; 30: 3901-3914. PMID: 37185408. Free PMC article.
Presented by
Dr. Cord Langner, Graz, and Dr. Hans Peter Gröchenig, St. Veit / Glan, Austria.