-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
April 2025
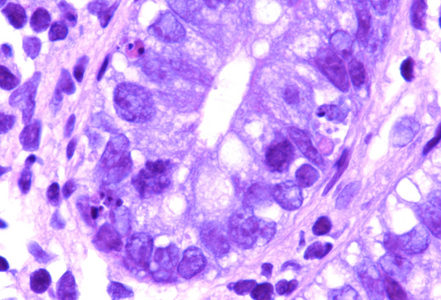
A colonic biopsy from a 76-year-old male patient with hepatocellular carcinoma.
Diagnosis
Colitis assoiated with Tremelimumab/Durvalumab treatment.
Comment
A 76-year-old male patient with unresectable multifocal hepatocellular carcinoma was put on treatment with tremelimumab/durvalumab (two new immune checkpoint inhibitors (ICIs), according to the HIMALAYA trial). Immediately after initiation of therapy (during the first course), severe diarrhoea and acute renal failure occurred (grade 3 toxicity), prompting cessation of treatment. Colonoscopy was performed and showed severe colitis in the inspected areas. Biopsies were taken.
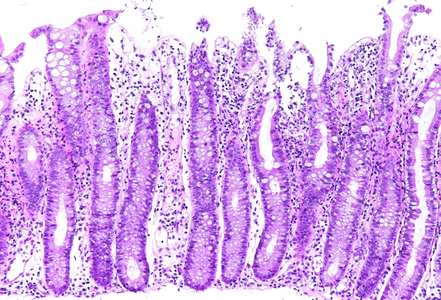
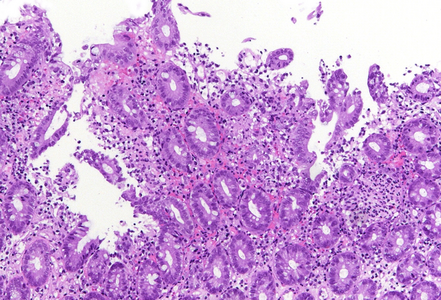
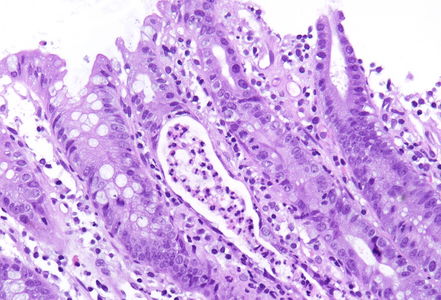
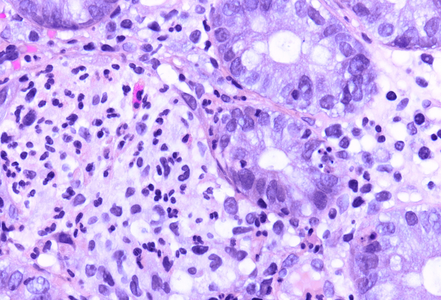
Histology showed preserved mucosal architecture (Panel A). Severe active inflammation was present, with crypt abscess formation and crypt rupture with cryptolytic granulomas and multifocal erosion (Panels B-D). Apoptosis was increased, sometimes with several apoptotic bodies within a single crypt (Panel E).
Immune checkpoint ihibitors are well known to induce gastrointestinal toxicity. Several patterns have been described, including active or focal active inflammation (with mild crypt architectural distortion depending on disease duration), intraepithelial lymphocytosis (celiac disease-like pattern or lymphocytic colitis-like pattern) and apoptotic excess as know from graft versus host disease. Often mixed patters are observed, and morphology may vary individually.
Colitis dues to combined treatment with tremelimumab/durvalumab has only been reported once. Remarkably, as in our case, sudden-onset diarrhoea occurred already during the first course of treatment. This is unusual, since in most cases of ICI-related toxicity side affects occur with some delay, often weeks or even months after treatment initiation.
For further reading
- Terrin M, Migliorisi G, Dal Buono A, Gabbiadini R, Mastrorocco E, Quadarella A, Repici A, Santoro A, Armuzzi A. Checkpoint Inhibitor-Induced Colitis: From Pathogenesis to Management. Int J Mol Sci. 2023 Jul 15;24(14):11504. doi: 10.3390/ijms241411504. PMID: 37511260; PMCID: PMC10380448.
- Abe H, Endo K, Kuroda H, Oikawa T, Abe T, Ito A, Suzuki A, Yoshida Y, Kakisaka K, Matsumoto T. Immune checkpoint inhibitor-associated colitis in unresectable hepatocellular carcinoma: two cases of early onset after treatment with durvalumab plus tremelimumab. Clin J Gastroenterol. 2024 Apr;17(2):307-310. doi: 10.1007/s12328-023-01901-y. Epub 2024 Jan 7. PMID: 38185741.
- Radulescu L, Crisan D, Grapa C, Radulescu D. Digestive Toxicities Secondary to Immune Checkpoint Inhibition Therapy - Reports of Rare Events. A Systematic Review. J Gastrointestin Liver Dis. 2021 Dec 21;30(4):506-516. doi: 10.15403/jgld-3671. PMID: 34752584.
Presented by
Dr. Jasmina Redzepagic, Sarajevo, Bosnia and Herzegovina, and Dr. Cord Langner, Graz, Austria.